2024-05-27



Target

Deal Size

Buyer

This week, an alternative asset transaction is worth noting. Royalty Pharma has invested in Cytokinetics’ Aficamten, a first-in-class drug intended for hypertrophic cardiomyopathy (HCM) currently in phase III clinical trials, with a $300M upfront payment and $275M in milestone payments. As the only drug clinically verified to be effective for HCM, it has good safety and tolerability and can effectively improve the ejection fraction of patients.
Royalty Pharma’s business model is similar to that of a fund-like company, profiting by investing in back-end sales royalties or asset rights. The company mainly invests in products post-PoC (Proof of Concept) or already approved, with relatively low scientific risk. In 2023, the company’s revenue reached $2.35 billion, and its representative investment products include blockbuster drugs such as Revlimid and Eylea.
The trend of not investing in company equity but in company assets has also begun to emerge in China, e.g. the GLP-1 transaction recently signed between Hengrui and Bain Capital and other investment institutions, which is very similar to Royalty Pharma’s business model.
Peter Zhang
Partner, YAFO Capital



Target

Deal Size

Buyer

本周值得关注的是一项另类资产交易。Royalty Pharma以3亿美金预付款和2.75亿美金里程碑的形式投资了Cytokinetics的Aficamten,一款拟用于肥厚性心肌病(HCM)的首创药物,目前在进行三期临床实验。作为唯一一款被临床证明有效的HCM药物,具有良好的安全性和耐受性,可以有效提高患者的射血分数。
Royalty Pharma的业务模式类似于一种基金制公司,通过投资pipeline后端销售分成或者资产的权益来获利。 公司主要投资PoC后或者已上市的产品,有比较低的科学风险。2023年,公司收入达到23.5亿美金,其代表性的投资产品包括Revlimid、Eylea等超级重磅炸弹药物。
不投资公司股权而是公司资产的趋势在中国也已经开始出现,比如恒瑞和贝恩资本等投资机构在近期达成的GLP-1交易,与Royalty Pharma的业务模式非常相似。
Peter Zhang
Partner, YAFO Capital

1. Summary of the Week
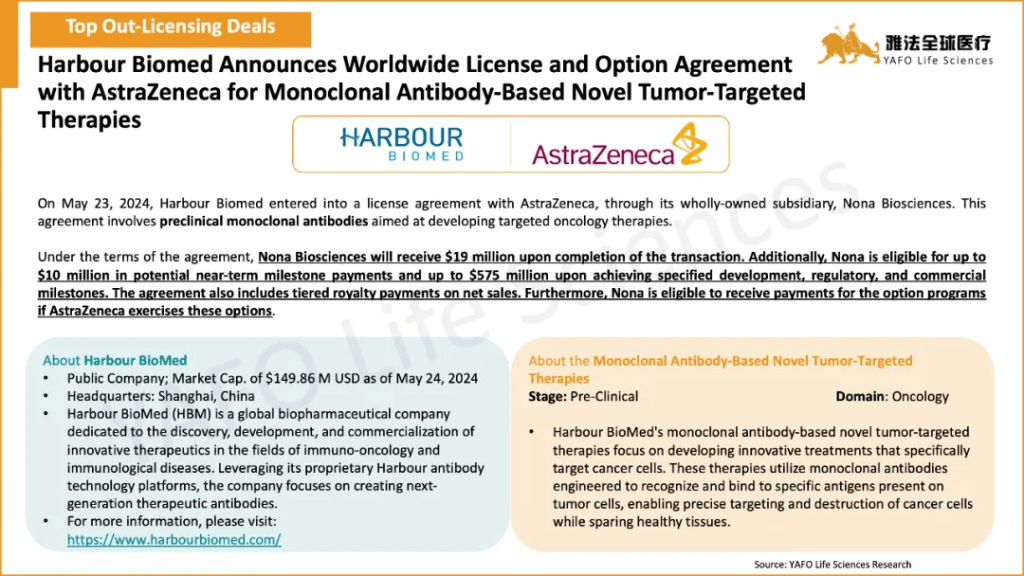
Between May 18 and May 24, a total of 15 licensing and cooperation deals were finalized worldwide. In China’s biotech sector, there were 2 out-licensing deals and 1 in-licensing deal. Notable agreements included a $604 million licensing and option deal between Harbour BioMed and AstraZeneca for a pre-clinical oncology asset, a licensing and cooperation agreement between Degron Therapeutics and Takeda for the GlueXplorer® platform.
Overall, 12 global licensing and cooperation deals were completed. The most significant was a cooperation agreement between Aktis Oncology and Eli Lilly, valued at $1,160 million with an upfront payment of $60 million.
5月18日-24日,全球共达成15项资产授权与合作交易。中国医药市场共达成3项交易,包括2项出海交易和1项国内交易。值得关注的交易包括和铂医药与AstraZeneca之间达成的价值6.04亿美元的临床前肿瘤资产授权和选择权交易,以及达歌生物与Takeda之间就GlueXplorer®平台签订的授权与合作交易。
国际市场上,共签署了12项资产授权与合作交易。其中最值得关注的交易是Aktis Oncology与Eli Lilly之间达成的多个靶点创新核药合作交易,首付款6000万美元,总金额11.6亿美元。
2. Licensing Deals
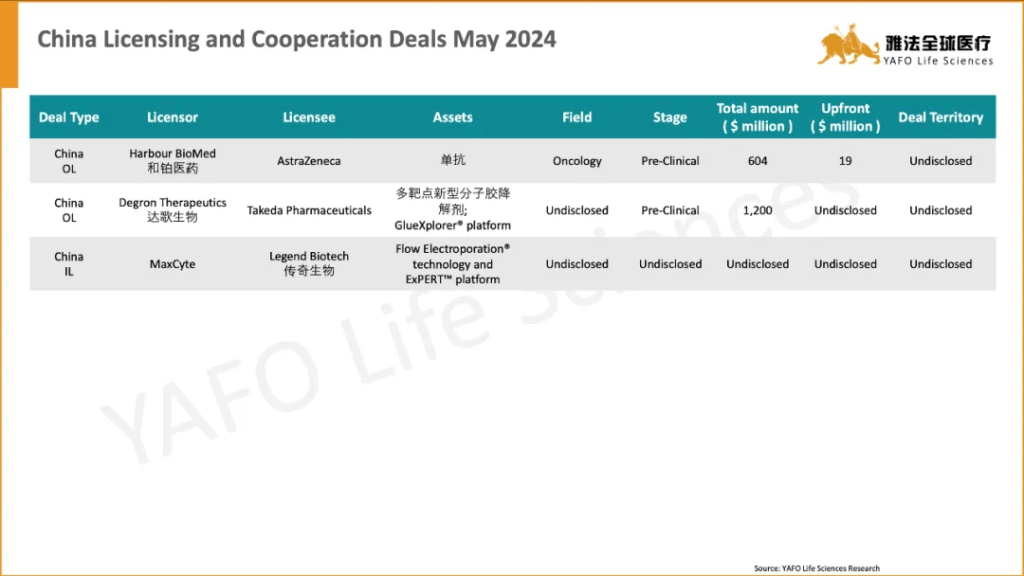
2a. China section


2b. Global section


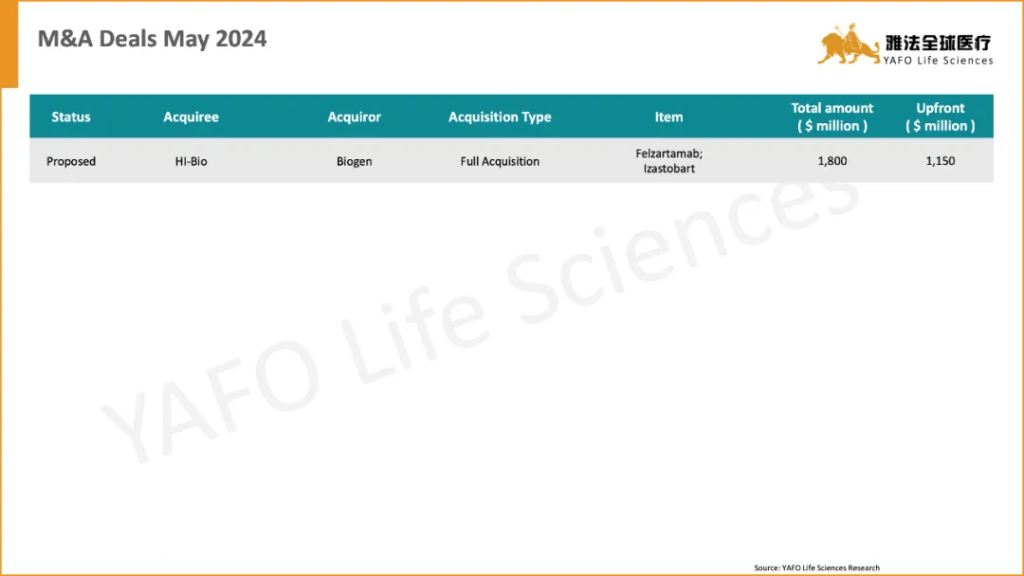
3. M&A Deals
4. Top Deals of the Year 2024


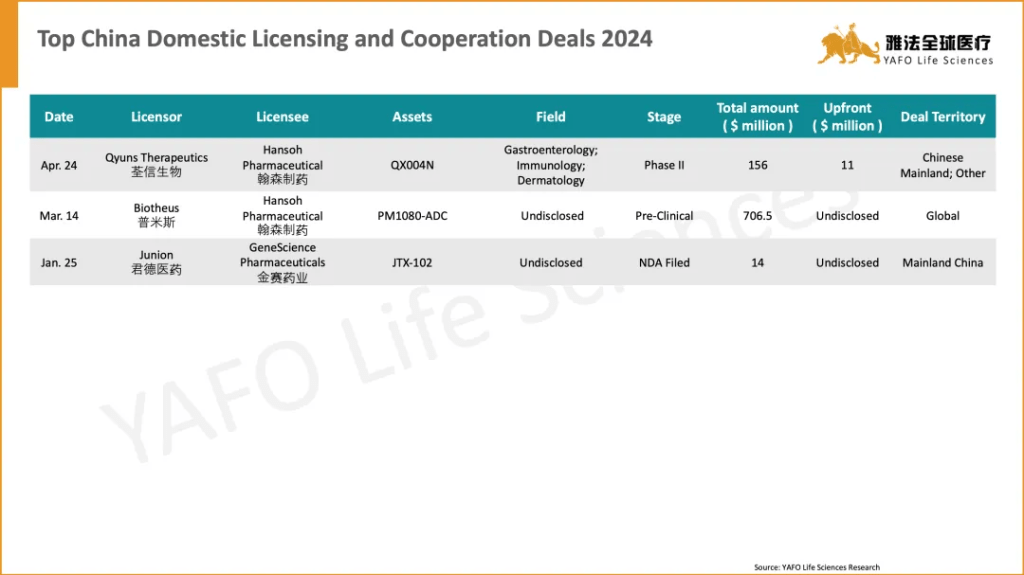

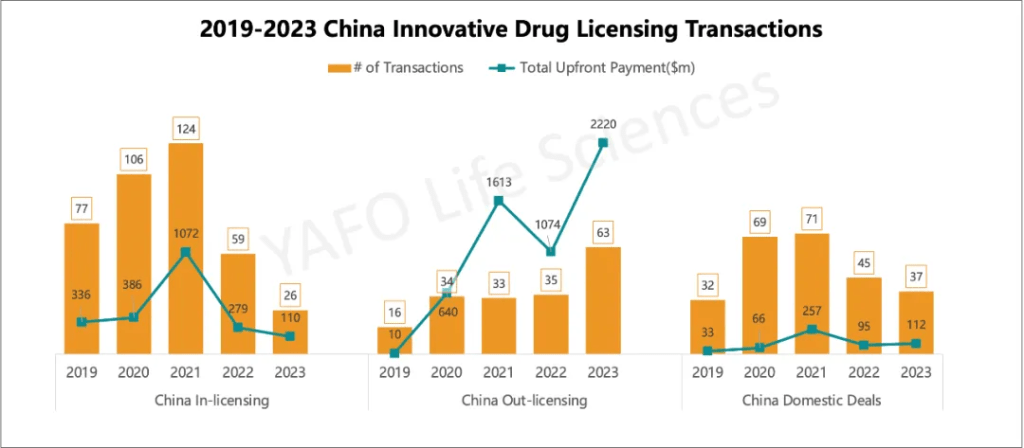
5. 2019-2023 China Innovative Drug Licensing Transactions
About YAFO Capital
Founded in 2013, YAFO Capital is a Shanghai based boutique investment and advisory firm, with professional team in our China, U.S., and London offices. Partnering with Pharmaceutical companies, YAFO Fund mainly invests in global assets. YAFO Life Sciences is a leading advisory boutique focused on asset transactions. YAFO has built a strong proven track record and closed dozens of in-licensing and out-licensing transactions with global pharma and biotech companies. YAFO has been ranked as the No. 1 advisor for China cross border licensing transactions in the past three years. For more information, please visit http://www.yafocapital.com
雅法资本成立于2013年,作为新型投资和投行咨询机构,致力于中国及海外生物医药项目的投资、融资服务、产品引进和资产孵化等。雅法在生物医药跨境授权及并购业务领域过往三年交易数量排名第一。旗下雅法基金联合药企进行资产投资和并购,雅法全球医疗专注于医药产品跨境及国内授权交易。基于雅法在全球广泛的人脉与资源网络,在过去十年成功推进了大量的海外项目进入中国市场并协助多个中国产品完成海外授权。雅法拥有经验丰富的全球交易团队,覆盖美国、日本 、欧洲等全球主要医药创新区域。核心合伙人均为华尔街资深投行人士或具有跨国药企经历,为客户交易提供强力支持。雅法总部位于上海,在伦敦、洛杉矶、东京、米兰、剑桥等地均设有分部。
ACCESS CHINA

Event Name : ACCESS CHINA Partnering Forum @BIO
Date & Time : June 3-20, 2024
Venue : San Diego & Online
Content : Keynote Speeches, Panel Discussion, Virtual Roadshows, Dinner Reception, 1X1 meetings
Scale : Expected 1000 participants Online, 150 participants onsite; 100 company roadshows
Participants : Pharma/Biotech senior management and BDs.
Registration Link : https://jinshuju.net/f/AqkB9m