
1.Summary of the week
In the first half of December 2023, there are total of 21 Licensing Deals globally including Licensing deals in China. In particular, there are 4 out-licensing deals; 1 in-licensing deal; 2 domestic deals; and 14 overseas deals. The largest deal out-licensing of the year is the deal between Systimmune and Bristol-Myers Squibb amounting $800 million upfront payment including $7.6 billion milestone payment with the total potential consideration of up to $8.4 billion.
1a. China section
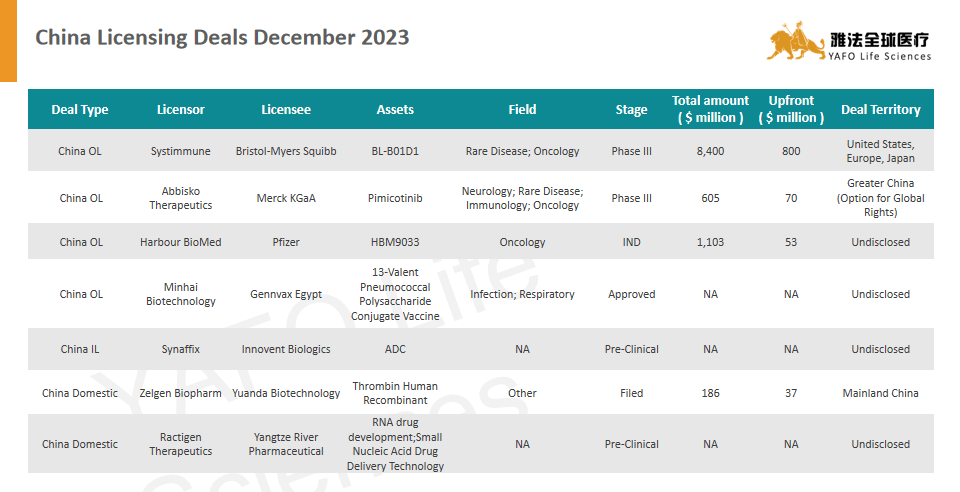
Systimmune and Bristol-Myers Squibb sign licensing deal for oncology candidate BL-B01D1
Bristol Myers Squibb (BMY) and Redmond’s SystImmune have formed an exclusive partnership to co-develop and market the oncology candidate BL-B01D1 in the United States. BL-B01D1, created by SystImmune, is an EGFRxHER3 bispecific antibody-drug conjugate (ADC) that holds promise as a pioneering treatment. It’s presently undergoing assessment in a worldwide early-stage study to gauge its safety and effectiveness in people with metastatic or unresectable non-small cell lung cancer (NSCLC).
Under the agreement, Bristol Myers Squibb will initiate the partnership with an $800 million upfront payment to SystImmune. The two companies will collaborate on global development expenses and share profits and losses in the United States. Additionally, SystImmune stands to gain up to $500 million in contingent near-term payments and could receive further milestone payments totaling up to $7.1 billion upon hitting specific development, regulatory, and sales benchmarks, resulting in a potential overall consideration of $8.4 billion.
SystImmune will retain exclusive rights for development and commercialization in Mainland China, offering Bristol Myers Squibb a royalty on net sales in that region. Meanwhile, Bristol Myers Squibb takes on sole responsibility for the candidate’s development and commercialization in all other parts of the world. SystImmune will receive a tiered royalty based on net sales outside the United States and Mainland China.
Abbisko Therapeutics and Merck KGaA sign licensing deal for Pimicotinib
On December 4, 2023, Abbisko Therapeutics announced a licensing deal with Merck, a prominent science and technology company in Germany. Merck will exclusively market products containing pimicotinib (ABSK021) in specific regions (Chinese mainland, Hong Kong, Macau, and Taiwan). Abbisko retains the exclusive rights to develop pimicotinib within these areas. Merck also gains an option for global commercial rights, with the chance to co-develop pimicotinib for additional uses. Abbisko will receive a $70 million upfront payment, and if Merck opts for global rights, an additional fee. The total potential payment, including milestones, amounts to $605.5 million, along with a double-digit percentage royalty on yearly net sales.
Pimicotinib, a novel CSF-1R inhibitor developed by Abbisko Therapeutics, is a new, orally administered, highly selective, and potent small molecule. It has received special designations such as breakthrough therapy designation (BTD) and Priority Medicine (PRIME) designation from China’s NMPA, the U.S. FDA, and the EMA. These designations aim to facilitate its use in treating TGCT patients unsuitable for surgery. This compound is undergoing the first global Phase III clinical trial for TGCT, concurrently conducted in China, the U.S., Canada, and Europe. As of the current announcement date, no highly selective CSF-1R inhibitor has obtained approval in China.
The biopharmaceutical company Harbour BioMed has entered into a licensing agreement with Pfizer for HBM9033
Harbour BioMed’s subsidiary, Nona Biosciences, has recently finalized an exclusive licensing deal with Pfizer Inc. for the worldwide clinical development and commercialization rights of HBM9033, an antibody-drug conjugate (ADC) targeting MSLN.
HBM9033, potentially Best-in-Class, is an antibody-drug conjugate (ADC) designed to specifically target human MSLN which is an identified as a tumor-associated antigen (TAA) found in several types of solid tumors. The monoclonal antibody (mAb) within HBM9033 is entirely human and originates from the Harbour Mice® platform. This antibody possesses precisely balanced characteristics, demonstrating diminished binding to shedding MSLN (sMSLN) while retaining robust binding and internalization capabilities when interacting with membrane-bound MSLN.
As per the agreement, Nona Biosciences stands to receive a sum totaling up to $53 million in upfront and near-term payments. There’s also potential for additional payments amounting to a substantial $1.05 billion upon successful attainment of specific developmental and commercial milestones. Moreover, Nona Biosciences remains eligible for tiered royalties on net sales, ranging from high single digits to high teens.
Minhai Biotechnology signed an authorization agent and technology transfer agreement with Gennvax Egypt for dual-vector 13-valent pneumonia vaccine
Beijing Minhai Biotechnology Co., Ltd. signed the “13-valent Pneumococcal polysaccharide conjugate Vaccine Authorization Agency and technology transfer Agreement” with Gennvax Egypt, an Egyptian vaccine company. The two companies reached a commercial cooperation on agent licensing and technology transfer of the world’s first dual-carrier 13-valent pneumococcal polysaccharide conjugate vaccine independently developed and produced by Minhai Biotechnology. Both companies will jointly promote the approval of the above-mentioned finished vaccines in Egypt, as well as the local bulk solution packaging.
1b. Global section
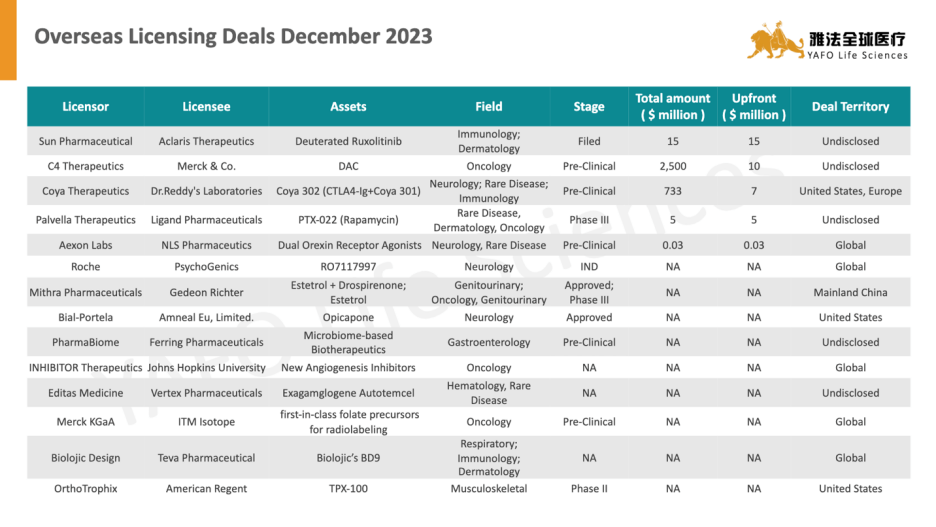
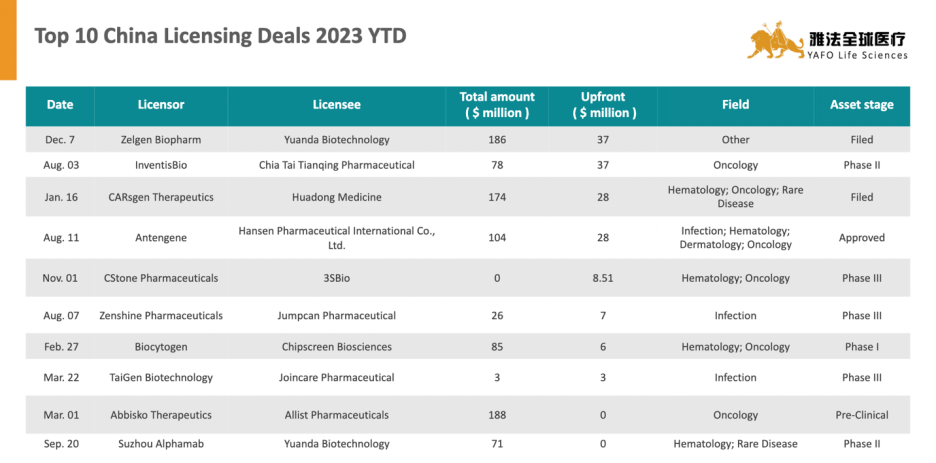
2. Top Deals of the Year 2023
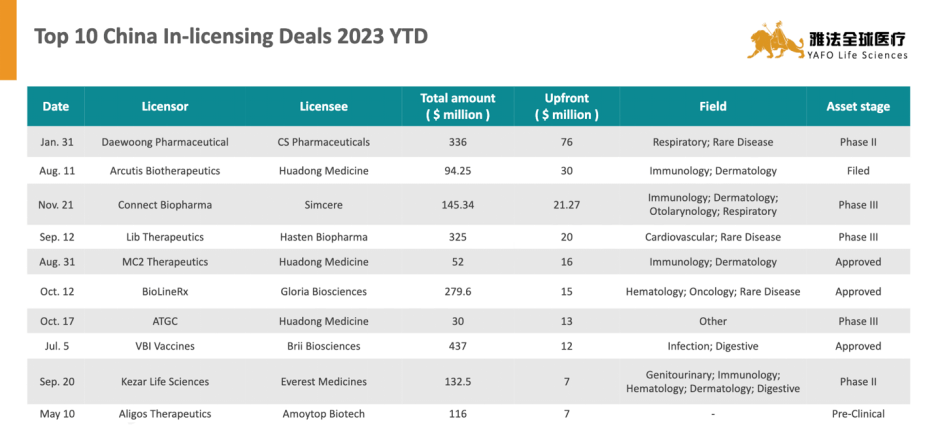
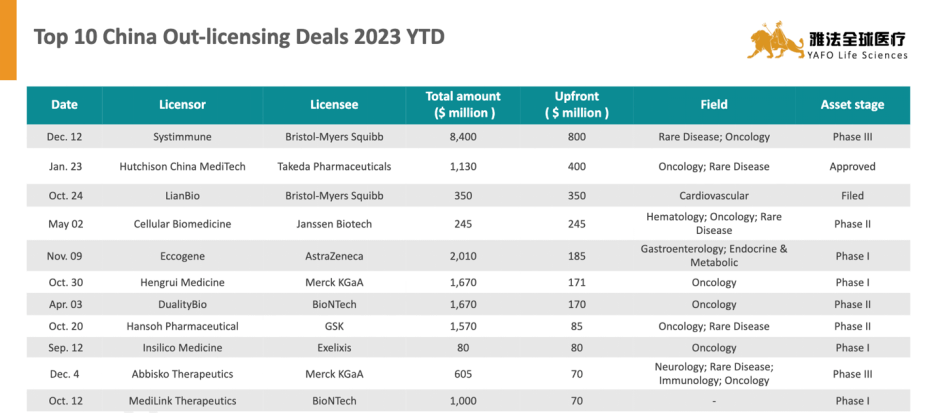
关于雅法资本(YAFO Capital)
雅法资本成立于2013年,作为新型投资和投行咨询机构,致力于中国及海外生物医药项目的投资、融资服务、产品引进和资产孵化等。雅法在生物医药跨境授权及并购业务领域过往三年交易数量排名第一。旗下雅法基金联合药企进行资产投资和并购,雅法全球医疗专注于医药产品跨境及国内授权交易。基于雅法在全球广泛的人脉与资源网络,在过去十年成功推进了大量的海外项目进入中国市场并协助多个中国产品完成海外授权。雅法拥有经验丰富的全球交易团队,覆盖美国、日本 、欧洲等全球主要医药创新区域。核心合伙人均为华尔街资深投行人士或具有跨国药企经历,为客户交易提供强力支持。雅法总部位于上海,在伦敦、洛杉矶、东京、米兰、剑桥等地均设有分部。
Founded in 2013, YAFO Capital is a Shanghai based boutique investment and advisory firm, with professional team in our China, U.S., and London offices. Partnering with Pharmaceutical companies, YAFO Fund mainly invests in global assets. YAFO Life Sciences is a leading advisory boutique focused on asset transactions. YAFO has built a strong proven track record and closed dozens of in-licensing and out-licensing transactions with global pharma and biotech companies. YAFO has been ranked as the No. 1 advisor for China cross border licensing transactions in the past three years. For more information, please visit http://www.yafocapital.com
活动预告–ACCESS CHINA

《ACCESS CHINA 药通中国》2024交易论坛(冬季)将在JPM会议期间举办,形式包括线上路演、现场1X1会议、及东西方交流晚宴(East-Meet-West Dinner),为有海内外授权需求的药企提供与全球合作伙伴一对一、面对面的沟通机会。本次药通中国@JPM2024论坛将于2024年1月在旧金山及线上举行,我们将邀请来自欧美、日韩以及新兴市场(包括东南亚、中东、拉美)等地区的BD人员参会,帮助中国药企走向更广阔的市场。因席位有限,为了确保您能够参加本次盛会,我们诚邀您尽早报名。最终参会确认以主办方参会通知函为准。期待与各位同仁相聚旧金山!
了解更多报名信息请点击:持续招募中… 药通中国@JPM 2024携手全球伙伴共筑医疗创新未来
We are honored to invite you to join our “ACCESS CHINA Partnering Forum” during JPM week in January 2024. During this event, we will host Online Showcases for international and China biotech companies for presentations, Dinner Reception to gather senior management from both international and China pharma for networking, and 1×1 meetings to YAFO clients for partnering opportunities. ACCESS CHINA Partnering Forum will help biotechs/pharmas efficiently present their assets in front of 500+ healthcare companies and VCs online. Audiences can have access to innovative biopharmas and healthcare products from worldwide. We look forward to welcoming you at our Partnering Forum in January!
For more information, please visit: https://biotochina.org/2023/11/10/access-china-partnering-forum-jpm-2024/

扫码报名
Registration