2024-07-01

1. Executive Summary of the Month
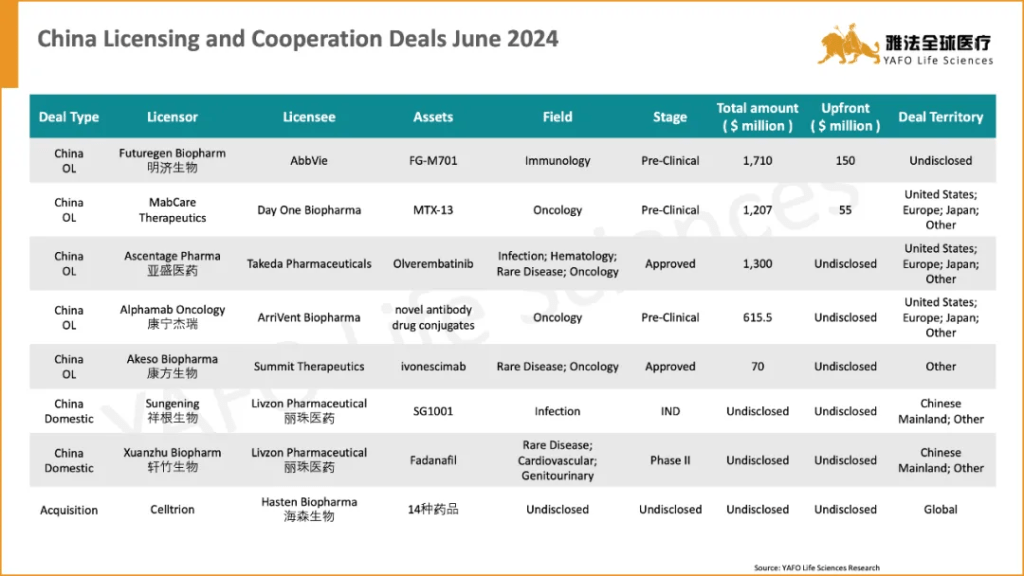
In June, a total of 36 licensing and cooperation deals were signed globally, marking a significant decrease compared to the previous month. Within the China Biotech Industry, 8 deals were signed: specifically, 5 out-licensing deals, 2 domestic licensing deals, and 1 acquisition deal. The most notable deal of the month is the out-licensing agreement between FuturegenBiopharm and AbbVie, valued at $1,710 million, which includes an upfront payment of $150 million.
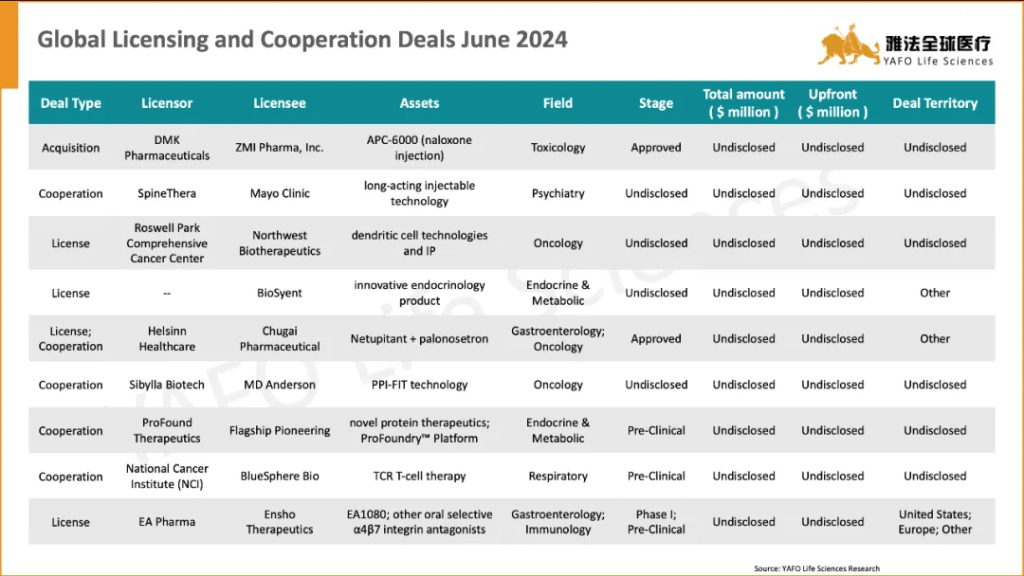
On a global scale, 28 licensing and cooperation deals were signed. The most significant of these is the license and investment deal between QurAlis and Eli Lilly for pre-clinical stage assets, valued at $622 million, with an upfront payment of $45 million.
2024年6月,全球医药市场共签署了36项资产授权和合作协议,较5月份的63项有显著减少。其中,中国医药市场达成8项交易,包括5项出海交易、2项国内交易和1项引进交易。本月中国市场最重要的交易是艾伯维和明济生物之间就后者一临床前资产达成的授权交易,首付款1.5亿美元,总金额17.1亿美元。
国际市场上,6月份共签署了28项资产授权和合作协议。最大的一笔交易是QurAlis与与Eli Lilly之间就前者多个临床前资产达成的授权交易,首付款4500万美元,总金额6.22亿美元。


Target
Deal Size

Buyer

On June 13th, AbbVie made a $150 million upfront payment to FutureGen to introduce FG-M701, an antibody pipeline for treating IBD targeting TL1A. As a next-generation TL1A treatment solution, it offers better therapeutic efficacy and dosing frequency, and has the potential to become a Best-in-Class (BIC).
TL1A, a very hot target over the past two years, saw Merck acquired Prometheus for $10.8 billion in 2023, while Roche introduced a TL1A pipeline incubated by Roivant for $7.1 billion. It is worth noting that TL1A is not the leading pipeline of FutureGen, and it is expected to enter clinical trials this year.
AbbVie, with Humira, has a significant advantage in the IBD field. This transaction will be its strategy to cope with the patent cliff of Humira in IBD. Compared to TNFα, TL1A has higher specificity for IBD diseases. In addition to this target, others including RIPK1, SMAD7, Toll-like receptors, and microbiome therapies could potentially disrupt the current standard of care (SoC) in this field.
Peter Zhang
Partner, YAFO Capital



Target
Deal Size

Buyer

6月13日,艾伯维以1.5亿美金的首付款从明济生物(FutureGen)引进了FG-M701, 一款治疗IBD的TL1A抗体管线。作为下一代TL1A治疗方案,该管线有着更好的疗效以及给药频次,有成为BIC的潜在可能。
TL1A作为过去两年非常火热的靶点,Merck在2023年以108亿美金并购了Promethus, 罗氏则是以71亿美金的价格引进了Roivant孵化的一款TL1A管线。值得注意的是,TL1A并不是明济最成熟的管线,FG-M701预计今年将进入临床试验。
艾伯维坐拥修美乐,在IBD领域有着巨大优势,这个交易将是其应对修美乐进入专利悬崖后对IBD的应对策略。对比TNFα, TL1A对IBD疾病的特异性更强。除了该靶点,包括RIPK1, SMAD7, TOLL样受体等靶点,微生物治疗等不同方案都有可能颠覆该领域的SoC。
Peter Zhang
Partner, YAFO Capital



Target

Deal Size

Buyer

This week’s hot transaction we have selected is the collaboration between Roche and Ascidian based on RNA exon editing technology signed on June 18th. Roche will pay Ascidian an upfront and milestone payment of up to $1,842 million to obtain the global exclusive license for Ascidian technology platform, EXTEND™ RNA exon editing technology, applied in the field of central nervous system (CNS) diseases. The two parties will jointly develop drugs based on the technology for the treatment of neurological diseases such as Parkinson’s disease and Alzheimer’s disease.
RNA exon editing is an emerging gene editing technology that can precisely regulate gene expression by changing the splicing process of RNA, and it is expected to be used to treat a variety of diseases. Compared with traditional gene editing technologies such as CRISPR, RNA exon editing can achieve higher precision and safety by optimizing gRNA design and the combination of various editing enzymes, and it does not make permanent modifications to DNA.
Through the cooperation with Roche, Ascidian can continuously evaluate and optimize the risks and efficiency of its large fragment editing technology through multi-omics, and at the same time, it is expected to explore more applications in other diseases based on the maturity of Roche’s delivery technology platform in the future.
Peter Zhang
Partner, YAFO Capital



Target

Deal Size

Buyer

本周热点交易我们选择了Roche与Ascidian于6月18日达成的基于RNA外显子编辑技术的合作。Roche将支付Ascidian最高18.42亿美元的首付款和里程碑付款,以获得Ascidian开发的技术平台EXTEND™RNA外显子编辑技术在中枢神经系统(CNS)疾病领域的全球独家许可权。双方将共同开发基于该技术的药物,用于治疗帕金森病、阿尔茨海默病等神经系统疾病。
RNA外显子编辑是一种新兴的基因编辑技术,通过改变RNA的剪接过程,可以精确地调控基因表达,有望用于治疗多种疾病。与传统的基因编辑技术如CRISPR相比,RNA外显子编辑可以通过优化gRNA设计,多种编辑酶的组合获得更高的精确性和安全性,而且不会对DNA进行永久性修改。
通过与罗氏的合作,Ascidian可以通过多组学持续评估及优化其大片段剪辑技术的风险和效率,同时未来有望根据罗氏的递送技术平台的成熟在其他疾病领域发掘更多的应用。
Peter Zhang
Partner, YAFO Capital

2. Licensing Deals



2a. Out-Licensing Deals

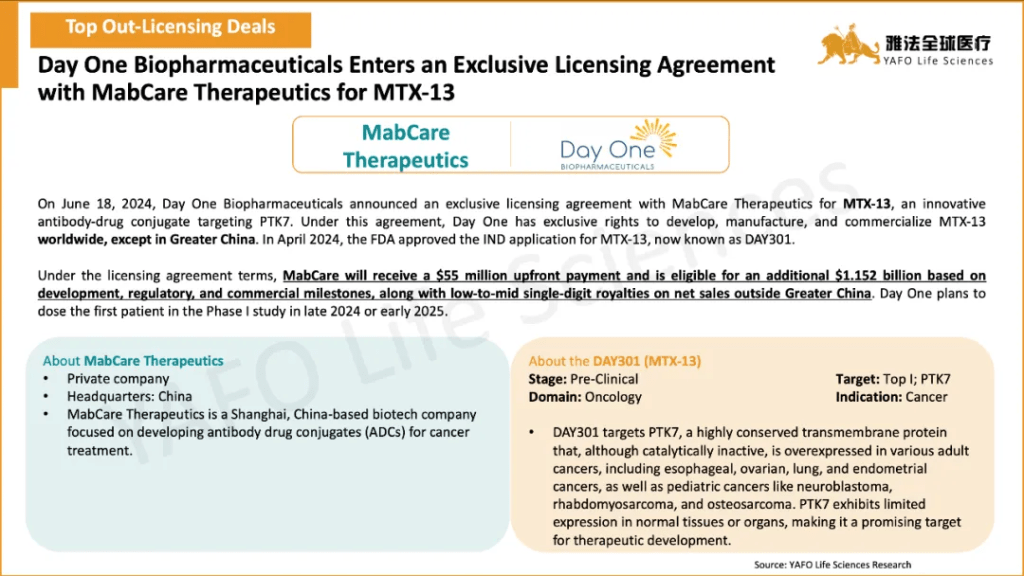
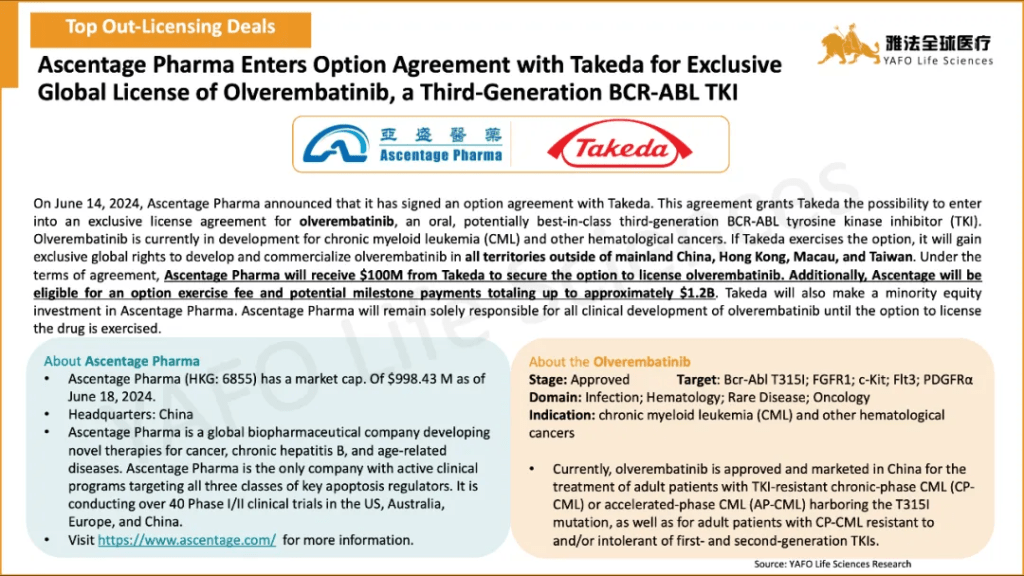

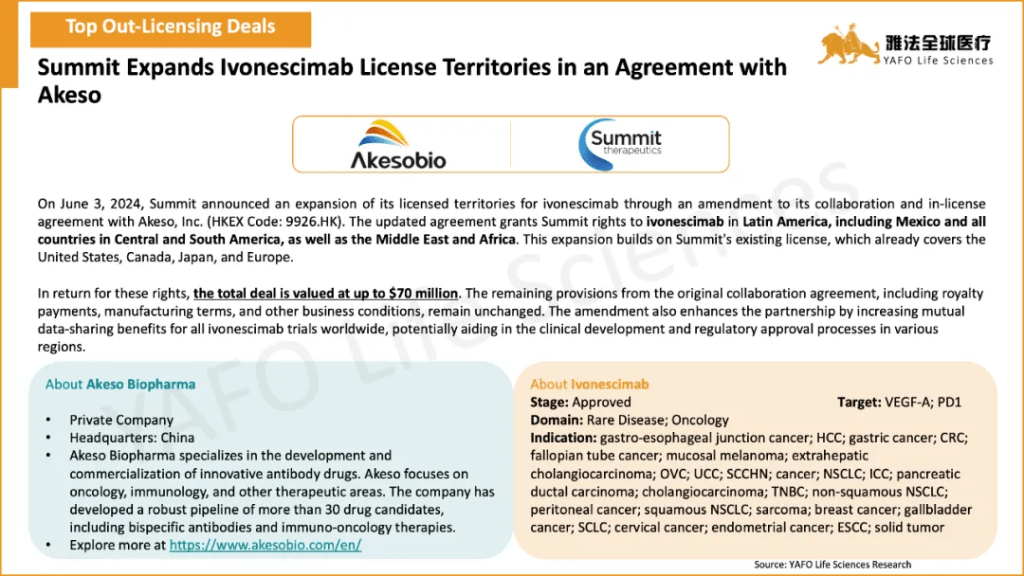
2b. Domestic Licensing Deals


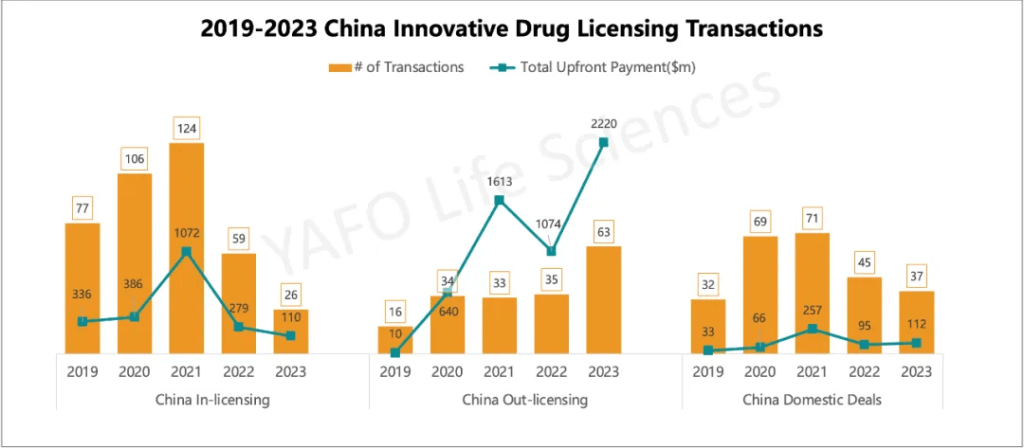
3. 2019-2023 China Innovative Drug Licensing Transactions
About YAFO Capital
Founded in 2013, YAFO Capital is a Shanghai based boutique investment and advisory firm, with professional team in our China, U.S., EU and SEA offices. Partnering with Pharmaceutical companies, YAFO Fund mainly invests in global assets. YAFO Life Sciences is a leading advisory boutique focused on asset transactions. YAFO has built a strong proven track record and closed dozens of in-licensing and out-licensing transactions with global pharma and biotech companies. Over the past five years, YAFO has been ranked as the No. 1 advisor for China cross border licensing transactions. For more information, please visit http://www.yafocapital.com
雅法资本成立于2013年,作为新型投资和投行咨询机构,致力于中国及海外生物医药项目的投融资、资产跨境交易和资产孵化等。旗下雅法基金联合药企进行资产投资和并购,雅法全球医疗专注于医药产品跨境及国内授权交易。基于雅法在全球广泛的人脉与资源网络,在过去十年成功推进了大量的海外项目进入中国市场并协助多个中国产品完成海外授权。雅法拥有经验丰富的全球交易团队,覆盖美国、日本 、欧洲等全球主要医药创新区域。核心合伙人均为华尔街资深投行人士或具有跨国药企经历,为客户交易提供强力支持。雅法总部位于上海,在美国、欧洲、东南亚等地均设有分部。雅法在生物医药跨境授权及并购业务交易数量连续多年排名第一。
ACCESS CHINA

Event Name : ACCESS CHINA Networking & Gathering @BIO
Date & Time : June 2-6, 2024
Venue : San Diego
Content : Reception, 1X1 meetings
Participants : Pharma/Biotech senior management and BDs.
Registration Link : https://jinshuju.net/f/AqkB9m?x_field_1=BIO